Speeding the Development and Approval of Breakthrough Drugs
In 2012, to address rising concerns about the time required to develop vital medications, Congress passed legislation creating the Breakthrough Therapy Designation (BTD). To qualify for this designation, proposed drugs must treat serious conditions and fill unmet needs, and pharmaceutical companies must provide substantial preliminary evidence that the new drugs represent major advances over existing therapies. Firms submit their BTD request after completing Phase I or II trials — the former typically a test for safety and dosage, the latter, using a larger sample, for safety and efficacy.
In Regulatory Incentives for Innovation: The FDA’s Breakthrough Therapy Designation (NBER Working Paper 30712), Amitabh Chandra, Jennifer Kao, Kathleen L. Miller, and Ariel D. Stern compare the time to approval of similar types of drugs for the periods before and after the implementation of the legislation. They find that the program shortened the clinical development time of drugs by nearly a quarter, without any evidence of reduction in safety.
Legislation designed to make major advances in therapeutic drugs available more quickly cut clinical development times by nearly 25 percent.
Firms that create drugs that qualify for BTD receive a host of benefits designed to reduce the time needed to reach patients. Regulators offer guidance on streamlining the development process, including help in defining the target population, defining an appropriate control group in clinical research, and identifying measures of success. The Food and Drug Administration (FDA) provides feedback on drug development plans before they are formally submitted. Its scientists contribute their expertise on what went right and wrong in previous projects as well as a big-picture view that reaches from the laboratory to the factory to the pharmacy shelf.
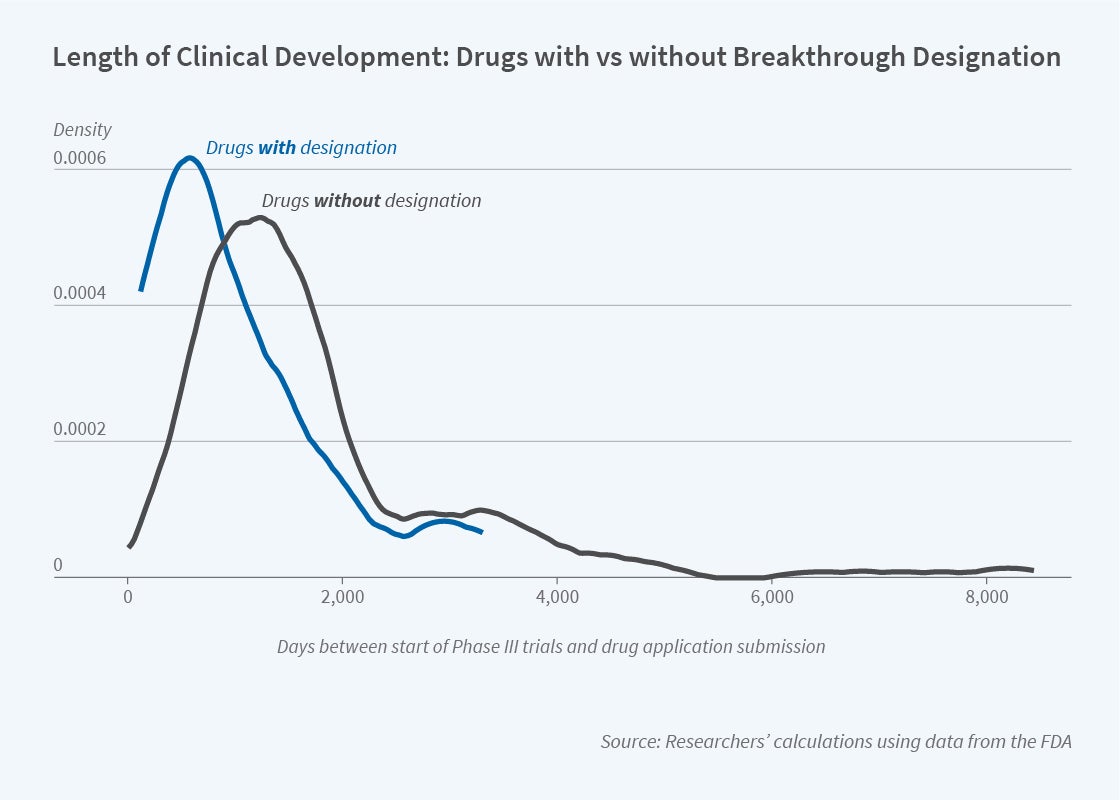
The BTD program does not reduce evidentiary requirements, but it speeds development of new drugs by making the process more efficient and facilitating reduced trial complexity. Focusing on time spent in late-stage trials, which is the most costly stage of drug development, the researchers estimate that the BTD leads to a 23 percent decline in the time spent between the start of late-stage trials and the submission of a drug application. BTD products spent an average of 2.74 years (32.9 months) in the last phase of pre-approval clinical trials. Previous estimates suggest that each additional month for late-stage trials costs $671,000, suggesting that the BTD program may save drug manufacturers $5 million.
The BTD program disproportionately accelerated development of drugs by relative newcomers, suggesting that engagement with regulators can help to close the gap between established and less-experienced firms. The researchers suggest that the program also has benefits for the FDA, because knowledge gleaned in the BTD context “could well provide the basis for better or more efficient decisions for non-BTD drugs.” More generally, they conclude that the BTD program shows that strong science combined with dedicated regulatory resources can accelerate the development and commercialization of valuable new products.
— Steve Maas


